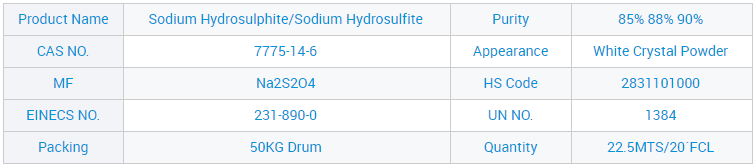
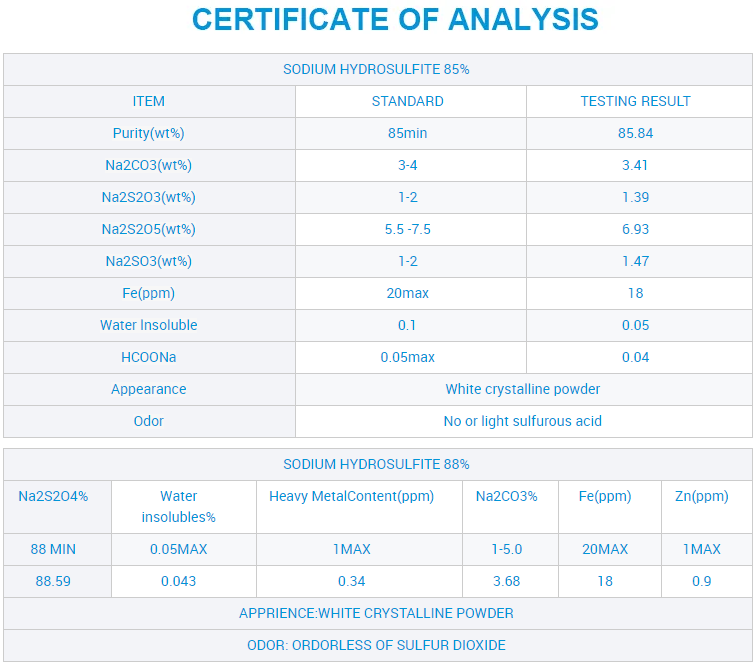
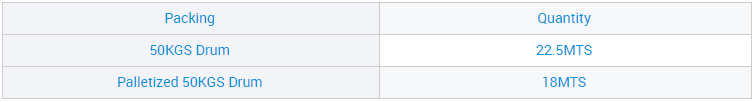
Ixabiso le-CAS 7775-14-6 Sodium Hydrosulfite lokuNcedisa
Iimveliso zethu zichongiwe kakhulu kwaye zithembekile ngabathengi kwaye ziya kwanelisa iminqweno ekhulayo yezoqoqosho nezentlalo ye-CAS 7775-14-6 Ixabiso leSodium Hydrosulfite lokuNcedisa, "Ukwenza Iimveliso Ezikumgangatho Ophezulu" yinjongo engunaphakade yenkampani yethu. Senza imizamo engapheliyo yokufezekisa injongo ethi "Siza Kuhlala Sihambelana Nexesha".
Iimveliso zethu zichongiwe kakhulu kwaye zithembekile ngabathengi kwaye ziya kwanelisa iminqweno ehlala ikhula yezoqoqosho nezentlalo, Ukuze ukwazi ukusebenzisa ulwazi oluvela kurhwebo lwamazwe ngamazwe, samkela abathengi abavela kuyo yonke indawo kwi-intanethi nangaphandle kwe-intanethi. Nangona sinezisombululo ezisemgangathweni esizibonelelayo, inkonzo yokubonisana esebenzayo neyanelisayo inikezelwa liqela lethu leenkonzo ezithengisa emva kokuthengisa. Uluhlu lweemveliso kunye neeparameter ezipheleleyo kunye naluphi na olunye ulwazi ziya kuthunyelwa kuwe ngexesha elifanelekileyo ngemibuzo yakho. Ngoko ke qiniseka ukuba unxibelelana nathi ngokusithumelela ii-imeyile okanye usitsalele umnxeba ukuba unemibuzo malunga nenkampani yethu. Ungafumana ulwazi lwedilesi yethu kwiphepha lethu lewebhu uze kwinkampani yethu ukuze ufumane uphando lwemveliso yethu. Siqinisekile ukuba sizimisele ukwabelana ngempumelelo kunye nokudala ubudlelwane obuqinileyo bentsebenziswano namaqabane ethu kule marike. Sijonge phambili kwimibuzo yakho.








Imibuzo ebuzwa qho
Ngaba ufuna uncedo? Qiniseka ukuba undwendwela iiforam zethu zenkxaso ukuze ufumane iimpendulo kwimibuzo yakho!
Ngaba singayiprinta ilogo yethu kwimveliso?
Kakade, singakwenza oko. Sithumelele nje uyilo lwelogo yakho.
Ngaba uyazamkela iiodolo ezincinci?
Ewe. Ukuba ungumthengisi omncinci okanye uqalisa ishishini, ngokuqinisekileyo sizimisele ukukhula nawe. Kwaye sijonge phambili ekusebenzeni nawe ubudlelwane bexesha elide.
Kuthekani ngexabiso? Ngaba ungayenza ifikeleleke kancinci?
Sisoloko sithatha inzuzo yomthengi njengeyona nto iphambili. Ixabiso liyaxoxiswana phantsi kweemeko ezahlukeneyo, siyakuqinisekisa ukuba ufumana ixabiso elikhuphisanayo.
Ngaba niyazibonelela ngeesampuli zasimahla?
Kuyaxatyiswa ukuba ungasibhalela uphononongo oluhle ukuba uyayithanda imveliso kunye nenkonzo yethu, siza kukunika iisampulu zasimahla kwi-odolo yakho elandelayo.
Ngaba uyakwazi ukunikezela ngexesha?
Ewe kona! Sikhetheke kakhulu kule ntambo kangangeminyaka emininzi, abathengi abaninzi benza isivumelwano nam kuba siyakwazi ukuhambisa iimpahla ngexesha kwaye sigcine iimpahla zisemgangathweni ophezulu!
Ndingandwendwela inkampani yakho kunye nomzi-mveliso eTshayina?
Ewe. Wamkelekile kakhulu ukutyelela inkampani yethu eZibo, eTshayina. (Iiyure ezili-1.5 ukusuka eJinan)
Ndingayifaka njani iodolo?
Ungasithumela nje umbuzo kuye nawuphi na ummeli wethu wentengiso ukuze ufumane ulwazi oluneenkcukacha zeodolo, size sichaze inkqubo yeenkcukacha. Iipropati zeKhemikhali zeSodium Hydrosulfite
a. Iarhente Yokunciphisa Enamandla: I-Rongalite ineempawu zokunciphisa ezinamandla kwaye idla ngokuxutywa ibe yi-sodium sulfite kunye ne-sodium sulfate.
b. Ukungazinzi kunye nokubola: I-Rongalite ayizinzanga ngokwemvelo kwaye iyabola phantsi kweemeko ezithile:
I-oxidation ekhawulezayo emoyeni: 2Na₂S₂O₄ + O₂ → 2Na₂SO₄ + 2SO₂↑
I-Sodium Hydrosulfite Reaction kwisisombululo samanzi: 2Na₂S₂O₄ + H₂O → Na₂S₂O₃ + 2NaHSO₃
Ukubola emoyeni omanzi: 2Na₂S₂O₄ + O₂ + 2H₂O → 4NaHSO₄
Ukubola ngokukhawuleza kumaqondo obushushu >90°C: 2Na₂S₂O₄ → Na₂SO₃ + Na₂S₂O₅
Ukutsha kunye nokutsha okuzenzekelayo kumaqondo obushushu angaphezu kwama-250°C.
c. Ukubola kweSodium Hydrosulfite kwi-Acidic/Basic Media:
Ibola ngokukhawuleza kwi-asidi: Na₂S₂O₄ + H₂SO₄ → Na₂SO₄ + SO₂↑ + S + H₂O
Izinzile kakhulu kwi-alkaline media, kodwa iyabola kwi-alkali enamandla: 3Na₂S₂O₄ + 6NaOH → 5Na₂SO₃ + Na₂S + 3H₂O













